
2020.12.03
The Pyeongtaek Bio Plant of Hanmi Pharm. has established a facility for producing the finished products other than the culturing and refining of the microorganisms in the Second Factory.
The manufacturing of the inoculation share of 20 million doses of the gene vaccine that had been cultured with the microorganisms a week is possible.
The speed of the production is 10 times faster and more convenient than the culturing of the animal cells that is utilized by a majority of the consigned production facilities domestically and overseas.
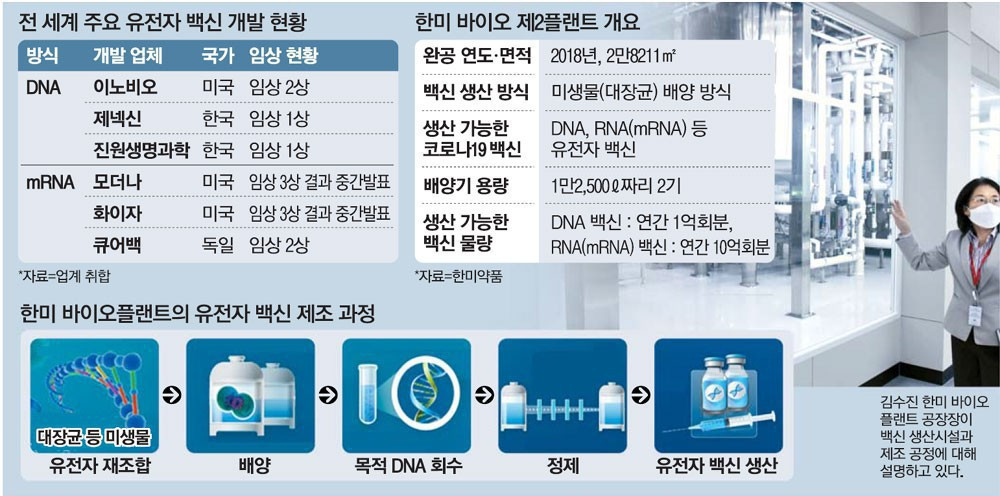 “At the Hanmi Bio Plant, the production of the vaccine of a maximum of 1 billion doses annually is possible. It could play a major role of enabling all of the citizens to quickly get out of the COVID-19 threat by efficient mass producing the vaccine as a request for the consigned production (CMO) of the vaccine comes in.”
Kim, Soo-jin, the manager of the Hanmi Pharm. Bio Plant located in the Pyeongtaek City, showed the confidence in this way regarding the vaccine production capability. Here, the bio plant facilities, including the Factory 1, the Factory 2, and the Management Building, have taken their places.
Especially, regarding the Factory 2 (the total ground area of 28,211㎡– around 85,000 pyeong), with the height of 6 floors, of which the construction was completed 2 years ago, it has been evaluated as the optimal conditions for a vaccine production base as it is fully equipped with the facility capable of culturing and refining 20,000ℓ of microorganism culture. When one enters the Factory 2, a culturing machine (a bio-reactor) that boasts of the height of 10m and the capacity of 12,500ℓ, which reminds of a giant water purifier barrel, catches the eye first. After culturing the colon bacilli in the culturing machine, by going through the process of collection and purification and the others, the gene vaccine (the DNA•mRNA vaccine) can be produced. The Executive Director Kim, Soo-jin, who is the sole woman Plant Manager of the Bio Plant in Korea, introduced “At the Factory 2 here, through the process of culturing the microorganism (the colon bacillus) for one week, the culture fluid of around 20,000ℓ can be obtained. In the case of the DNA vaccine, the amount of the inoculation share of 2 million doses can be manufactured. And, in the case of the RNA (mRNA) vaccine, the amount of the inoculation share of 20 million doses can be manufactured.” If these are converted into the annual unit, regarding the DNA vaccine, 100 million doses a year could be achieved. And, regarding the mRNA vaccine, the inoculation share of the vaccine of 1 billion doses a year can be produced. These are the sizes.”
The Director Koh, Seon-jin, who is in charge of the production at the Hanmi Bio Plant, said, “In order to end the COVID-19 pandemic, from hundreds of millions to billions of people must be inoculated at the same time. The plants that satisfy the condition of having to produce the nucleic acid (DNA, RNA), which is the raw material medicine of the gene vaccine, by a large amount in a short time period are a minimum number in the entire world to the extent of being counted on the fingers.” And the Director Koh emphasized by saying, “The competitiveness of the Factory 2 is that it can produce the gene vaccine, of which the demand has been rapidly increasing recently, in a large amount through the culturing of the microorganism.”
It is the explanation of the Director that, regarding the gene vaccine, rather than using the animal cells, it must be produced through the culturing of the microorganism, including the colon bacillus and the others of the like, in order for the production speed to be faster by 10 times and in order for the efficiency and the convenience to be higher. According to Hanmi Pharm., although there are many consigned bio medicine production corporations that have the large-sized culturing facilities, because the production of the antibody raw material medicine is the main purpose, they have the culturing facilities that are based on the animal cells. In the case of making a gene vaccine by utilizing the animal cells, it takes considerable time (around one month in order to culture the cells only).
Also, regarding the animal cells of which the tissue compositions are complicated, after the culturing, the process of the re-combination of the genes is difficult. And the production yield, too, is low.
On the other hand, if a microorganism (the colon bacillus) of which the tissue composition is simple is utilized, it takes only around several days for the culturing. And, by re-combining the genes of the colon bacillus cells, the vaccine can be produced right away. It is said that, if the colon bacillus is utilized, the mass production of the gene vaccine is possible at a maximum of 10 times faster than the method that utilizes the animal cells.
It is the explanation of Hanmi Pharm. that Celltrion and Samsung Biologics, which are the representative consigned production companies in Korea, and SK Bioscience and GC Pharma Corporation, too, which won the consigned COVID-19 vaccine production contracts from a global pharmaceutical company very recently, have the vaccine production facilities that are based on the fertile chicken eggs or the animal cells.
In the situation in which the quarantine authority has been proceeding with the vaccine purchase negotiations with the multinational pharmaceutical companies, the Factory Manager Kim emphasized, “Rather than the government directly purchases the vaccine, signing an introduction of technology contract and increasing domestic consigned production scale are more efficient ways to secure the quantity of vaccine.” Once the government sets out and makes a contract for the consigned production of a COVID-19 vaccine with a global pharmaceutical company, the Hanmi Bio Plant will perform its role as an outpost for the production.
It was confirmed that as the production capability of Hanmi Bio Plant has drawn much attention, many global pharmaceutical companies that have been developing the gene vaccines have tried to reach Hanmi Pharm. recently.
Hanmi Pharm. said, “The situation is that we have been proceeding with the discussions with plural pharmaceutical companies.” A related source at Hanmi Pharm. expected, “once a final production contract gets concluded with the gene vaccine development companies from which the inquiries have been coming in at the present, the worry, too, regarding the operation rate of the Hanmi Bio Plant will be relieved.”
Source: Maeil Business Newspaper.
“At the Hanmi Bio Plant, the production of the vaccine of a maximum of 1 billion doses annually is possible. It could play a major role of enabling all of the citizens to quickly get out of the COVID-19 threat by efficient mass producing the vaccine as a request for the consigned production (CMO) of the vaccine comes in.”
Kim, Soo-jin, the manager of the Hanmi Pharm. Bio Plant located in the Pyeongtaek City, showed the confidence in this way regarding the vaccine production capability. Here, the bio plant facilities, including the Factory 1, the Factory 2, and the Management Building, have taken their places.
Especially, regarding the Factory 2 (the total ground area of 28,211㎡– around 85,000 pyeong), with the height of 6 floors, of which the construction was completed 2 years ago, it has been evaluated as the optimal conditions for a vaccine production base as it is fully equipped with the facility capable of culturing and refining 20,000ℓ of microorganism culture. When one enters the Factory 2, a culturing machine (a bio-reactor) that boasts of the height of 10m and the capacity of 12,500ℓ, which reminds of a giant water purifier barrel, catches the eye first. After culturing the colon bacilli in the culturing machine, by going through the process of collection and purification and the others, the gene vaccine (the DNA•mRNA vaccine) can be produced. The Executive Director Kim, Soo-jin, who is the sole woman Plant Manager of the Bio Plant in Korea, introduced “At the Factory 2 here, through the process of culturing the microorganism (the colon bacillus) for one week, the culture fluid of around 20,000ℓ can be obtained. In the case of the DNA vaccine, the amount of the inoculation share of 2 million doses can be manufactured. And, in the case of the RNA (mRNA) vaccine, the amount of the inoculation share of 20 million doses can be manufactured.” If these are converted into the annual unit, regarding the DNA vaccine, 100 million doses a year could be achieved. And, regarding the mRNA vaccine, the inoculation share of the vaccine of 1 billion doses a year can be produced. These are the sizes.”
The Director Koh, Seon-jin, who is in charge of the production at the Hanmi Bio Plant, said, “In order to end the COVID-19 pandemic, from hundreds of millions to billions of people must be inoculated at the same time. The plants that satisfy the condition of having to produce the nucleic acid (DNA, RNA), which is the raw material medicine of the gene vaccine, by a large amount in a short time period are a minimum number in the entire world to the extent of being counted on the fingers.” And the Director Koh emphasized by saying, “The competitiveness of the Factory 2 is that it can produce the gene vaccine, of which the demand has been rapidly increasing recently, in a large amount through the culturing of the microorganism.”
It is the explanation of the Director that, regarding the gene vaccine, rather than using the animal cells, it must be produced through the culturing of the microorganism, including the colon bacillus and the others of the like, in order for the production speed to be faster by 10 times and in order for the efficiency and the convenience to be higher. According to Hanmi Pharm., although there are many consigned bio medicine production corporations that have the large-sized culturing facilities, because the production of the antibody raw material medicine is the main purpose, they have the culturing facilities that are based on the animal cells. In the case of making a gene vaccine by utilizing the animal cells, it takes considerable time (around one month in order to culture the cells only).
Also, regarding the animal cells of which the tissue compositions are complicated, after the culturing, the process of the re-combination of the genes is difficult. And the production yield, too, is low.
On the other hand, if a microorganism (the colon bacillus) of which the tissue composition is simple is utilized, it takes only around several days for the culturing. And, by re-combining the genes of the colon bacillus cells, the vaccine can be produced right away. It is said that, if the colon bacillus is utilized, the mass production of the gene vaccine is possible at a maximum of 10 times faster than the method that utilizes the animal cells.
It is the explanation of Hanmi Pharm. that Celltrion and Samsung Biologics, which are the representative consigned production companies in Korea, and SK Bioscience and GC Pharma Corporation, too, which won the consigned COVID-19 vaccine production contracts from a global pharmaceutical company very recently, have the vaccine production facilities that are based on the fertile chicken eggs or the animal cells.
In the situation in which the quarantine authority has been proceeding with the vaccine purchase negotiations with the multinational pharmaceutical companies, the Factory Manager Kim emphasized, “Rather than the government directly purchases the vaccine, signing an introduction of technology contract and increasing domestic consigned production scale are more efficient ways to secure the quantity of vaccine.” Once the government sets out and makes a contract for the consigned production of a COVID-19 vaccine with a global pharmaceutical company, the Hanmi Bio Plant will perform its role as an outpost for the production.
It was confirmed that as the production capability of Hanmi Bio Plant has drawn much attention, many global pharmaceutical companies that have been developing the gene vaccines have tried to reach Hanmi Pharm. recently.
Hanmi Pharm. said, “The situation is that we have been proceeding with the discussions with plural pharmaceutical companies.” A related source at Hanmi Pharm. expected, “once a final production contract gets concluded with the gene vaccine development companies from which the inquiries have been coming in at the present, the worry, too, regarding the operation rate of the Hanmi Bio Plant will be relieved.”
Source: Maeil Business Newspaper.